Reports of Serious Injuries After COVID Vaccines Near 112,000, as Pfizer Asks FDA to Green Light Shots for Kids 5 to 11
Data released Friday by the Centers for Disease Control and Prevention (CDC) showed that between Dec. 14, 2020 and Oct. 1, 2021, a total of 778,685 adverse events following COVID vaccines were reported to the Vaccine Adverse Event Reporting System (VAERS). The data included a total of 16,310 reports of deaths — an increase of 373 over the previous week.
There were 111,921 reports of serious injuries, including deaths, during the same time period — up 6,163 compared with the previous week.
Excluding “foreign reports” filed in VAERS, 593.728 adverse events, including 7,437 deaths and 47,455 serious injuries, were reported in the U.S. between Dec. 14, 2020 and Oct. 1, 2021.
Of the 7,437 U.S. deaths reported as of Oct. 1, 11% occurred within 24 hours of vaccination, 16% occurred within 48 hours of vaccination and 29% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 393.4 million COVID vaccine doses had been administered as of Oct. 1. This includes: 227 million doses of Pfizer, 152 million doses of Moderna and 15 million doses of Johnson & Johnson (J&J).
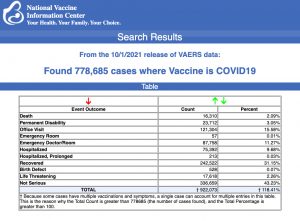
The data come directly from reports submitted to VAERS, the primary government-funded system for reporting adverse vaccine reactions in the U.S.
Every Friday, VAERS makes public all vaccine injury reports received as of a specified date, usually about a week prior to the release date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
This week’s U.S. data for 12- to 17-year-olds show:
- 21,298 total adverse events, including 1,284 rated as serious and 22 reported deaths. Two of the 22 deaths were suicides.
The most recent death involves a 16-year-old male (VAERS I.D. 1734141) who reportedly died from cardiac failure five days after receiving Pfizer’s COVID vaccine.
Other recent deaths include a 17-year-old male (VAERS I.D. 1689212) with cancer who was vaccinated April 17, tested positive for COVID on July 20, was hospitalized and passed away Aug. 29; and a 16-year-old female (VAERS I.D. 1694568) who died from a pulmonary embolism nine days after receiving her first Pfizer dose.
- 3,202 reports of anaphylaxis among 12- to 17-year-olds with 99% of cases
attributed to Pfizer’s vaccine. - 520 reports of myocarditis and pericarditis (heart inflammation) with 508 cases attributed to Pfizer’s vaccine.
- 114 reports of blood clotting disorders, with all cases attributed to Pfizer.
This week’s U.S. VAERS data, from Dec. 14, 2020 to Oct. 1, 2021, for all age groups combined, show:
- 19% of deaths were related to cardiac disorders.
- 56% of those who died were male, 43% were female and the remaining death reports did not include gender of the deceased.
- The average age of death was 72.8.
- Of the 2,935 cases of Bell’s Palsy reported, 50% were attributed to Pfizer vaccinations, 42% to Moderna and 8% to J&J.
- 648 reports of Guillain-Barré syndrome, with 40% of cases attributed to Pfizer, 32% to Moderna and 28% to J&J.
- 1,976 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 158,280 reports of symptoms of anaphylactic reactions with 43% of cases attributed to Pfizer’s vaccine, 49% to Moderna and 7% to J&J. An anaphylactic reaction may include various symptoms like skin rashes, nausea, vomiting, difficulty breathing or shock.
- 9,907 reports of blood clotting disorders. Of those, 4,286 reports were attributed to Pfizer, 3,595 reports to Moderna and 1,975 reports to J&J.
- 2,737 cases of myocarditis and pericarditis with 1,733 cases attributed to Pfizer, 888 cases to Moderna and 106 cases to J&J’s COVID vaccine.
Young mother pressured to receive COVID vaccine dies of vaccine-induced blood clots
Jessica Berg Wilson, a 37-year-old stay-at-home mother from Washington passed away suddenly on Sept. 7 from vaccine-induced thrombotic thrombocytopenia (VITT) — a rare, and sometimes fatal, blood-clotting condition — after receiving J&J’s COVID vaccine.
In exclusive interview w/ #TheDefender, Jessica Berg Wilson’s husband + uncle share devastating story of Jessica’s death, which was attributed to vaccine-induced thrombotic thrombocytopenia caused by J&J COVID vaccine.
SIGN UP: https://t.co/zL66EdwTnDhttps://t.co/pSnXQNCBCH
— Robert F. Kennedy Jr (@RobertKennedyJr) October 6, 2021
On Aug. 29, Jessica went to a Seattle pharmacy to get her COVID vaccine and was told she would be receiving J&J’s shot. She was “vehemently opposed” to taking the vaccine, “considering her stay-at-home mom status, state of good health and young age in conjunction with the known and unknown risk of an unproven vaccine,” her husband said.
But Jessica was pressured to get the vaccine due to a vaccine mandate at their child’s school requiring “room moms” who wished to serve in the classroom be fully vaccinated.
According to Jessica’s VAERS report (VAERS I.D. 1683324), she experienced blood clots in her ovarian and renal veins, and a brain hemorrhage that led to tissue damage. Although doctors tried to relieve the pressure on her brain by performing a craniotomy, they were unsuccessful.
Jessica was ultimately pronounced brain dead, removed from life support and passed away. Doctors confirmed the cause of death was VITT.
Pfizer asks FDA to authorize emergency use of its COVID vaccine for 5- to 11-year-olds
Pfizer and its German partner, BioNTech on Thursday asked the U.S. Food and Drug Administration (FDA) to authorize their COVID vaccine for emergency use for children 5 to 11 years old. The FDA advisory committee is scheduled to meet Oct. 26 to discuss Pfizer’s pediatric COVID vaccine.
FDA officials said once vaccine data for younger children was submitted, the agency could authorize a vaccine for younger children in a matter of weeks, but it would depend on the timing and quality of the data provided.
Pfizer and BioNTech submitted initial data to the FDA last month for a regimen of two 10-microgram doses in children — one-third the amount given to older patients — but had not formally requested authorization until now.
According to Pfizer’s Sept. 20 press release, the trial didn’t show the vaccine reduced hospitalizations or even mild cases. But it did reveal side effects generally comparable to those observed in participants 16 to 25 years of age.
Studies confirm Pfizer vaccine immunity wanes at 2 months
As The Defender reported, two studies published Wednesday in the New England Journal of Medicine confirm any immune protection offered by two doses of Pfizer’s COVID vaccine drops off after roughly two months.
A study from Israel covering 4,800 healthcare workers showed antibody levels waned rapidly after 2 doses of Pfizer’s COVID vaccine as Pfizer officially asked FDA to authorize its vaccine for children 5 to 11 years old.https://t.co/MqcsnQaKOz
— Robert F. Kennedy Jr (@RobertKennedyJr) October 8, 2021
A prospective longitudinal study from Israel covering 4,800 healthcare workers showed antibody levels waned rapidly after two doses of vaccine “especially among men, among persons 65 years of age or older and among persons with immunosuppression.”
A second study from Qatar looked at actual infections among the nation’s highly vaccinated population, who mostly received Pfizer’s COVID vaccine. Estimated effectiveness against SARS-CoV-2 infection was negligible for the first two weeks after the first Pfizer dose, increased to 36.8% in the third week after the first dose, and reached its peak at 77.5% in the first month after the second dose.
By months five five through seven, researchers said vaccine efficacy reached a low level of approximately 20%. Pfizer has consistently claimed the company’s own efficacy data demonstrate 95% efficacy against SARS-CoV-2, which was not observed in this study.
Sweden, Denmark and Finland pause Moderna vaccine over concerns of myocarditis
Sweden, Denmark and Finland will pause the use of Moderna’s COVID vaccine for younger age groups after reports of possible rare side effects, including myocarditis.
Sweden will pause Moderna’s COVID vaccine for people under age 30, while Denmark said it will halt use of Moderna’s vaccine in people under age 18.
SIGN UP #TheDefender: https://t.co/zL66EdwTnDhttps://t.co/gJfaBj466r
— Robert F. Kennedy Jr (@RobertKennedyJr) October 6, 2021
Finland on Thursday paused the use of Moderna’s COVID vaccine for younger males due to reports of myocarditis, joining Sweden and Denmark in limiting its use after a Nordic study involving Finland, Sweden, Norway and Denmark found men under the age of 30 who received Moderna’s vaccine had a slightly higher risk than others of developing myocarditis.
All four countries said they would instead give Pfizer’s vaccine to men born in 1991 and later, despite research that shows a similar risk of myocarditis associated with Pfizer’s vaccine.
Fully vaccinated patient sparks COVID outbreak among vaccinated population
A paper published Sept. 30, in Eurosurveillance showed a fully vaccinated patient in a hospital setting rapidly spread COVID to fully vaccinated staff, patients and family members — despite a 96% vaccination rate and use of full personal protective equipment.
Of the 42 cases diagnosed in the outbreak, 38 were fully vaccinated with two doses of Pfizer and BioNTech’s Comirnaty vaccine, one had received only one vaccination and three were unvaccinated.
Of the infected, 23 were patients and 19 were staff members. The staff all recovered quickly. However, eight vaccinated patients became severely ill, six became critically ill and five of the critically ill died. The two unvaccinated patients had mild COVID cases.
The authors said the study challenges the assumption high universal vaccination rates will lead to herd immunity and prevent COVID outbreaks, as 96.2% of the outbreak subjects were vaccinated, infection advanced rapidly and viral load was high.
Fully vaccinated countries had the highest number of new COVID cases
In a study published Sept. 30 in the peer-reviewed European Journal of Epidemiology Vaccines, researchers investigated the relationship between the percentage of population fully vaccinated and new COVID cases across 68 countries and 2,947 U.S. counties that had second dose vaccine, and available COVID case data.
The study found “no discernible relationship” between the percentage of population fully vaccinated and new COVID cases. In addition, the most fully vaccinated nations had the highest number of new COVID cases, based on the researchers’ analysis of emerging data during a seven-day period in September.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
The post Reports of Serious Injuries After COVID Vaccines Near 112,000, as Pfizer Asks FDA to Green Light Shots for Kids 5 to 11 appeared first on Children's Health Defense.
© 08 Oct 2021 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.






